
8 Reasons glucose is worth measuring
Glucose is only one piece of the metabolic health puzzle, but it’s an important one. Here’s why we believe in glucose monitoring for more people.
This is a companion article to Why glucose isn’t enough for good health, which outlines the reasons that glucose alone is necessary but not sufficient for metabolic health. Here, we explain why we advocate measuring it anyway.
Blood glucose monitoring has been an integral part of managing diabetes and limiting its secondary complications for the past half-century. In the past decade, support for continuous glucose monitoring (CGM) has increased exponentially, but it has only begun to expand into people without diagnosed metabolic impairment in the past few years.
More and more people without diabetes are seeking out CGMs to understand better how food and lifestyle choices impact their health. This trend toward opening access to personalized data, along with tools to help make that data actionable, is crucial to solving the metabolic health crisis. Today, more than a third of Americans are living with prediabetes. The condition increases the risk of diabetes, cardiovascular disease, and stroke. However, this kind of metabolic dysfunction can progress for years before people realize they have a problem—more than 80 percent of people with prediabetes are unaware that they have it. Those who develop diabetes account for $1 out of every $4 spent on healthcare in the US.
CGM measures the amount of glucose in the interstitial fluid just under your skin (it’s a close proxy for the amount of glucose in your blood) and, through intelligent software, shows you how your body is reacting to diet and lifestyle choices in near real-time. This kind of closed-loop feedback can empower us to make healthier decisions and, hopefully, reduce our risk of developing metabolic dysfunction. Many legitimate questions remain about the impact of CGM use in people without diagnosed metabolic impairment, but anecdotal evidence suggests that it could be genuinely transformative.
It’s important to note here that advocating for more visibility on one’s own data and adopting a diet that aims for stable blood sugar doesn’t mean obsessing over data or diet in unhealthy ways. Instead, the goal of this increased visibility into our body’s functioning—a term we call _biological observability—_is to learn more about yourself and what promotes long-term health for you. A glucose spike should never be the source of shame or anxiety but rather a learning opportunity. Similarly, we believe diet wars miss the point and do not advocate any specific dietary approach over another. Instead, we want to encourage mindful eating, avoiding processed foods and excess sugar, and incorporating personalized insights from data sources like CGM.

Ultimately, says Levels Advisor Dr. Sara Gottfried, we don’t have time to waste: “There are many limitations with glucose monitoring, but you have to start somewhere,” she says. “We’re in the midst of a serious crisis here, and the status quo isn’t cutting it. Glucose monitoring is a great place to start.”
Here are eight reasons why tracking glucose can be a helpful step in improving health and wellbeing.
1. CGM Reveals Where We Are on the Spectrum of Glucose Control
Today, there are three tests that a doctor might order to check how well your body processes glucose. The fasting plasma glucose (FPG) test looks at how much sugar is circulating in your blood after 8 or more hours without calories. The hemoglobin A1c test (HbA1c) measures your average blood glucose levels over the past three months. And the oral glucose tolerance test (OGTT) evaluates your body’s response to a high dose of sugar.
The tests all put people into one of three buckets—normal, prediabetes, or diabetes—depending on the result. So, for instance, a fasting glucose value below 100 mg/dL is considered “normal” according to the American Diabetes Association (ADA); anything between 100 and 126 mg/dL flags insulin resistance or prediabetes; and values higher than 126 mg/dL indicate diabetes.
But even within these groups, higher values are linked to increased health risks. For example, a person whose fasting glucose is 125 mg/dL is up to 1.5 times as likely to have a heart attack and has up to 1.2 times the risk of stroke compared to someone whose fasting glucose is 101 mg/dL—despite both people falling in the prediabetes range. And among people with “normal” fasting glucose, those with levels in the 91–99mg/dL range may have almost three times the risk of developing diabetes compared to those with levels less than 83 mg/dL. In his book Metabolical, Dr. Robert Lustig states, “Once fasting glucose rises over 100 mg/dL, metabolic syndrome is in full force. A fasting glucose of 90 mg/dL is already questionable.” These nuances are lost within the broad categories.
A prediabetes diagnosis is not just a wake-up call to inspire behavior change; it’s a sign that there is already metabolic dysfunction taking place. By lumping people into groups that define health states as buckets rather than a spectrum, today’s threshold-based diagnostics obscure developing problems. Your glucose control might be creeping from normal to impaired, with associated health impacts accumulating, but you may not be alerted until you tip into the next clinical bucket.
We can also look at metabolic dysfunction through a systems biology perspective, in which the body (the system) occupies different states, from a stable, healthy state to a pre-disease state when biomarkers may still be in a “healthy” range, but the system has less stability and can more easily tip into a third state of disease. By the time the system reaches this state, change again becomes more difficult. The point is that earlier detection of metabolic dysfunction offers a better chance of preventing metabolic disease.
The challenges of creating these diagnostic categories are highlighted in a 2020 study that compared the health outcomes of people diagnosed with prediabetes by either the ADA or the World Health Organization (WHO). The WHO threshold for prediabetes is a full 10 points higher than the ADA does, at 110 mg/dL. The study found that the extra leniency in the WHO’s criteria had a real effect. While about half of those diagnosed with prediabetes under the ADA criteria developed diabetes, almost two-thirds did under the WHO criteria.
These metabolic changes don’t happen overnight. Yet, for many people—especially those with “normal” glucose control—the tests simply aren’t performed often enough to detect and prevent the slide towards the next category or state. Only through continuous measurement can we get a clear picture of where we are on the metabolic spectrum.
With CGM, we can quit relying on sporadic, threshold-based testing and take ownership of our metabolic health.
2. Current Diagnostics Miss Glycemic Variability
Another drawback of the existing suite of tests is that they capture just a snapshot in time (fasting glucose, OGTT) or a flattened average (HbA1c). They all fail to capture the glucose fluctuations we experience throughout the day and night.
Understanding those oscillations is essential, though, because glycemic variability—the amount by which our blood glucose goes up and down throughout the day—is independently associated with poor health outcomes. Extensive evidence links glucose variability to cardiovascular complications in people with diabetes, mediated by the processes of oxidative stress and protein glycation.
This kind of variability may be more prevalent among people with “normal” glucose levels than previously realized. A 2018 study reported that 16 out of 20 participants who were classified as normal on standard clinical tests experienced severe post-meal glucose spikes that reached into the prediabetic range 15 percent of the time.
Excessive post-meal blood glucose spikes are an independent risk factor for a host of chronic conditions, including diabetes, cardiovascular disease, stroke, kidney failure, cancer, retina damage, and cognitive decline. Eating to promote stable blood sugar and developing metabolic flexibility is critical to supporting your long-term health and wellbeing.
CGM provides a dynamic, real-time view of blood sugar levels, helping us identify and avoid spike-triggering foods and behaviors and work towards maintaining stable glucose levels.
3. CGM Highlights Our Sugar Consumption
Sugar consumption in today’s standard American diet is as much as 10 times higher than it has been in the past.
The US Centers for Disease Control (CDC) recommends that no more than 10 percent of a person’s energy intake comes from added sugars. For a 2,000-calorie diet, that equates to about 50g or 12 teaspoons per day. The American Heart Association suggests the cap should be lower: a maximum of 6 percent of calories from added sugars (about 6 teaspoons for women, 9 for men). And the WHO thinks a daily limit of 5 percent “would be sensible.”
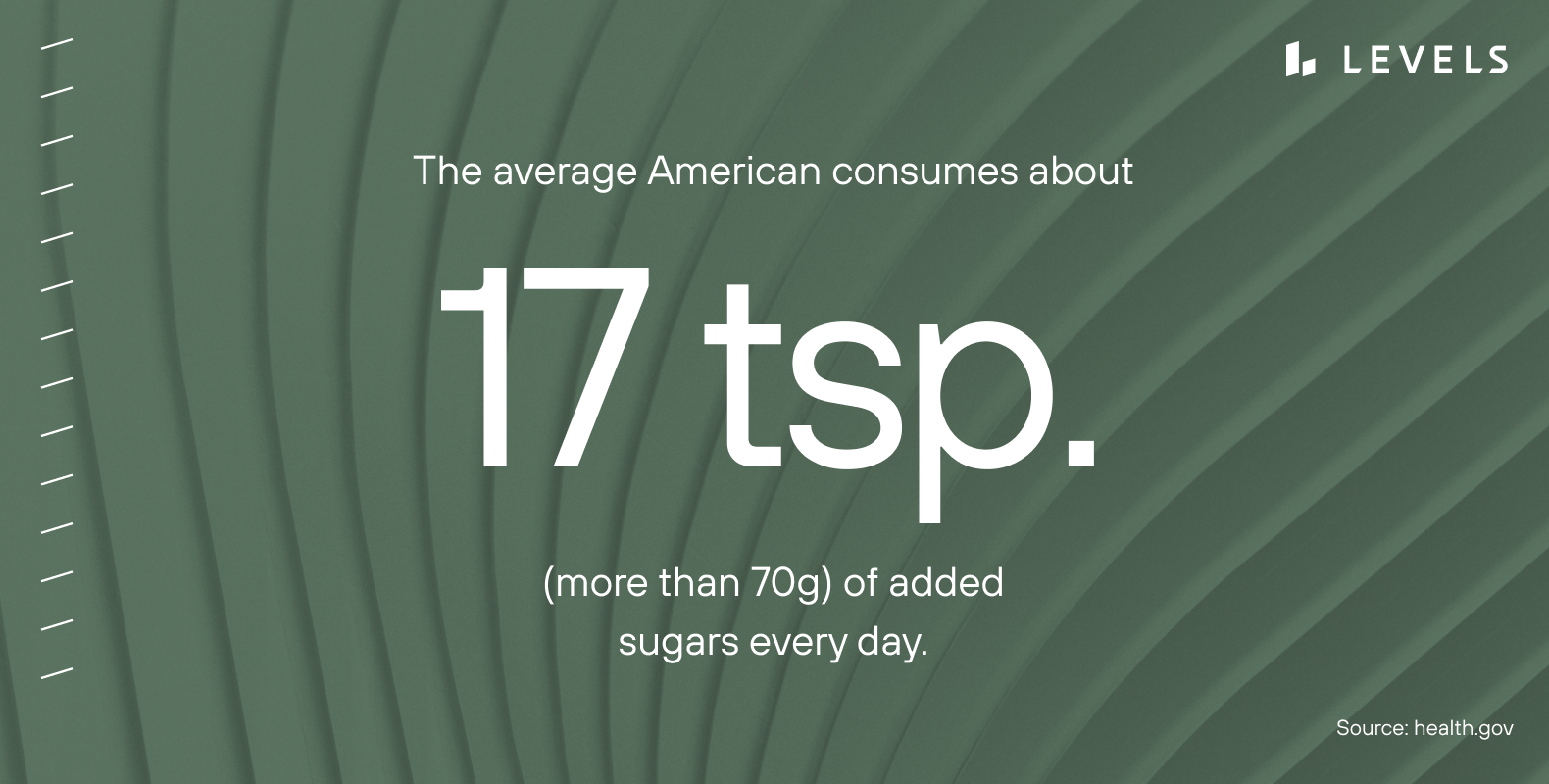
All of these are too high. Our bodies do not need any added sugar. We can get glucose from whole foods like fruits and vegetables and generate it through gluconeogenesis. We can also train our bodies to be metabolically flexible and burn ketones from fat instead of glucose.
Yet, the average American consumes about 17 teaspoons (more than 70g) of added sugars every day. Put another way, added sugars represent 13 percent of Americans’ daily energy intake, and fully two-thirds of us exceed even the 10 percent limit recommended by the CDC.
Besides the obvious culprits—soda, desserts, baked goods, candy—added sugar has found its way into all manner of everyday foods, such as salad dressings, ketchup, yogurt, and store-bought bread. Even the sugar we knowingly consume can be hard to grasp fully. Few of us can look at, say, a blueberry muffin and visualize the sugar it contains (39g, by the way—almost 10 teaspoons).
And it’s not just added sugars. Refined grains can also cause a glucose spike as they lack fiber, and the carbohydrates are quickly absorbed into our bloodstream. Certain starches, like white rice and some fruits, may also cause significant blood sugar spikes.
Tracking real-time glucose response provides clear evidence of the blood-sugar spiking carbohydrates we consume and the sometimes hidden ways we’re getting them, helping us make more mindful decisions.
4. Our Definition of “Normal” Glucose is Broken
A recent editorial in the journal JAMA made the argument that, where glucose is concerned, “normal is normal is normal.” In other words: unless you’re diagnosed with diabetes, you should consider yourself normal and needn’t fret over your glucose levels. However, the evidence appears to undermine that assertion: 122 million Americans—a staggering 45 percent of the adult population—either have diabetes or are on their way to developing it.
(On the topic of “normal,” It’s also worth noting that the study cited in the JAMA critique—which states that people without diabetes are in the normal range for glucose 96% of the time—55% of the study participants were under 25 and 37% were children (aged 6-18). Less than a third were in the 25-60 range. This underscores the point that “normal” depends on the population being studied.)
The accelerating rates of diabetes in this country and worldwide make clear that our current definition of normal is not sufficient.
In 1958, less than 1 percent of the US population was diagnosed with diabetes. By 2020, that number had ballooned more than ten-fold. This growth has happened despite impaired glucose tolerance (IGT) having been recognized as an intermediate state between normal and diabetic since 1979, and the broader classification of “prediabetes” gaining traction in the decades following.
Studies show that the majority of Type 2 diabetes is preventable. And yet, diabetes rates soar. By the time a person receives a prediabetes diagnosis, it may already be years since they had healthy metabolic function—and the ensuing cellular and systemic damage has already begun to take hold.
Alarm bells should be going off much louder, much sooner—which means our definition of normal urgently needs an overhaul. One study that used CGM to measure glucose response to a meal in people without diabetes found that the average post-meal glucose peak was 99 mg/dL plus or minus 10.5 mg/dL, far less than the IDF’s recommendation of 140 mg/dL.
“Not all people with prediabetes have high fasting glucose, so we want to look at the full dynamic range, including after meals,” says Dr. Gottfried. “Mainstream cut-off is <140 mg/dL at two hours after a meal. I like 115 mg/dL, but I also like to assess glucotype—i.e., how spikey you are.”
Widespread CGM use can help us redefine what normal should look like so that we can set better thresholds and catch developing metabolic disease sooner.
5. Understanding Individual Responses to Food Helps Us Tailor Our Choices
Dietary guidance focused on blood sugar often uses the concept of glycemic index (GI)—a score from 0 to 100 that indicates how fast a food’s carbs hit the bloodstream, and therefore how big of a spike they may cause. Low-GI foods—such as proteins, leafy greens, and nut butters—release their energy slowly and steadily, and produce only small fluctuations in blood glucose and insulin levels, while high-GI foods like white rice and candy send blood sugar soaring.
GI is a reassuringly simple system, and studies show that low-GI diets improve blood sugar control in both the short– and long term. But the tool has its limitations. Notably, a food’s GI describes the effect of eating an amount of a particular food that has 50g of accessible carbohydrates compared to eating an equivalent amount of carbs from a control food such as pure glucose. But this is not how we eat. We cook our foods and combine them in recipes and on our plates—all of which can alter how that food impacts our blood sugar.
Even more important: We’re not all built the same. The way our bodies react to food is influenced by our unique genome, epigenome, metabolome, microbiome, and even by transient factors like how stressed we are and how much sleep we’ve had. This means that while one person’s blood sugar may spike from a banana but remain relatively stable after eating a cookie, the inverse may be true of their neighbor.
GI can provide a ballpark idea of how a bowl of potatoes stacks up against a slab of tofu. But we can only glean insights into our highly-individualized responses to different foods under different circumstances by looking directly at our responses.
CGM delivers a personalized, data-rich picture of how our food and lifestyle choices impact our blood sugar, allowing us to eliminate the guesswork and find our optimal diet.
6. Glucose Impacts Wellbeing
In addition to the long-term metabolic health benefits, tracking glucose can improve daily quality of life.
Many of us have experienced the misery of a blood sugar crash—that spike and subsequent plunge in glucose levels following a carb-heavy meal or sugary snack. When we take in too many refined carbohydrates, it leads to an excess of glucose in our blood. Our pancreas tries to stabilize those blood sugar levels by secreting a surge of insulin to help move glucose into our cells or storage. However, too often, even after the excess glucose has been cleared, our insulin levels remain high, leading to an overcorrection and subsequent low blood sugar.
Unstable blood sugar is linked to depressed and anxious mood, “brain fog,” and impaired cognitive performance, disturbed sleep, chronic pain, and poor workout performance. Conversely, stable blood sugar can boost our energy levels, mood, productivity, and overall wellbeing.
Since we don’t all respond to foods precisely the same way, CGM can help us figure out our own personal spike triggers. Armed with that information, we can eat for stable glucose, avoid blood sugar crashes, and optimize our exercise, sleep and stress.
CGM is a wellbeing tool that can teach us how to manage our energy levels, mood, sleep, workout performance, and more.
7. Tracking Glucose Can Help Inspire Behavior Change
For more than three decades, doctors have been growing increasingly concerned about the global epidemic of metabolic dysfunction. In principle, metabolic syndrome—a cluster of factors that includes high blood sugar and dramatically raises the risk of cardiovascular disease, stroke, and diabetes—is preventable: eat whole foods, maintain a healthy weight, exercise, avoid smoking, and consume alcohol in moderation.
And yet, just 12 percent of Americans today are considered “metabolically healthy.” So far, we have failed to find an effective strategy to tackle metabolic dysfunction at scale.
In part, that’s because it’s hard for us to appreciate the transformative effects that our positive behaviors have at the cellular level. “You might exercise for a month, and nothing really seems to happen,” says Matthew Laye, PhD, who studies exercise metabolism, physiology, and human performance at The College of Idaho. “You haven’t lost any weight. You can’t clearly see that your health is any better. So, you stop.”
Exercise can feel like hard work, and when the rewards aren’t blindingly obvious, it’s easy to find excuses to avoid it. Meanwhile, we’re hard-wired to seek out the high-fat, high-sugar foods that set our brain’s reward center alight.

However, we know that people who wear fitness trackers tend to stick to programs designed to increase their activity level and that this translates to improved health outcomes. Wearables provide a “closed feedback loop” that gives us tangible, real-time proof of our good choices stacking up. That feedback feels like a reward, says Laye, which in turn boosts our motivation to stick at it.
Studies show that CGM is more effective than traditional finger-prick glucose monitoring (SMBG) at helping people with Type 2 diabetes improve their blood sugar control. What’s more, it can easily provide 50-100 times as many individual readings as even the most diligent SMBG user might collect in a given period—and with significantly less discomfort—making it an unparalleled source of insight for both the person wearing the device and their doctors.
By giving us a real-time view of what’s happening in our body, CGM can provide actionable data that makes it easier to change our behavior around diet, exercise, stress, and sleep. Biosensors and intelligent software can help us stay accountable to ourselves.
8. “Absence of Evidence is Not Evidence of Absence”
Although the evidence that people with “normal” blood sugar can benefit from tracking their glucose is primarily anecdotal so far, that’s not a reason to dismiss the idea. In fact, these initial success stories should be the impetus for us to investigate the potential of CGM’s broader-scale use more rigorously.
CGM technology has only been around for a couple of decades and remains prescription-only in the US. It makes sense that there are no completed large-scale studies of its utility in the general population so far. But don’t confuse absence of evidence for evidence of absence, says Peter Attia, MD.
“The randomized-controlled clinical trial data isn’t there [yet], but we still have a strong reason to believe it’s true,” writes Attia, on his website in response to the JAMA editorial, adding: “I do believe that relatively healthy people who track their glucose responses will fare better in the long run than those who don’t.”
Taking promising observations and subjecting them to scientific study is how new ideas become evidence-based medicine. In fact, for the first 10 years that CGM was available to people with diabetes, clinicians were doubtful that it could help people improve their glucose control. Today, the technology is widely recognized as beneficial for these populations, with organizations like the American Diabetes Association, the Endocrine Society, and the NIH publishing guidelines for its use in clinical practice.
However, to make a dent in the epidemic of metabolic dysfunction, we need a way to access and analyze our blood sugar profiles before diabetes takes hold. Medical science is just waking up to the potential of CGM to help guide and sustain healthy lifestyle choices among people who have not yet crossed that threshold. Several trials are now underway, with the first results expected at the end of 2021.
Conclusion
Today, we don’t know what’s happening inside our bodies, which means we are limited in what actions we can take to become healthier. We don’t have control because control flows downstream from observability. Continuous glucose monitoring is the current best window we have into our body’s response to diet and lifestyle. It’s a beginning but a powerful one. In part because the effects of dysregulated glucose are apparent. In part, because the amount of sugar we consume is very much in our control—the data is actionable. And in part, because it offers us a new way of thinking about using data to improve our health: real-time, individualized biological observability is the key to unlocking closed-loop behavior change that will reverse the metabolic health crisis at scale.

Why glucose isn't enough for good health
Glucose is an easy-to-measure marker of metabolic health, but stable blood sugar alone doesn't make you healthy. Here's why we have to get beyond glucose.

Take control of your metabolic health
Levels helps you see how food and lifestyle affect your health through macro tracking, habit-building, and customized insights and advice. Levels members can also incorporate biomarker data like real-time glucose and metabolic blood testing for an even more personalized experience. Click here to get started with Levels.





